I have been quite on a roll trying to work out these extremely complex chapters in your AP Chemistry curriculum and trying to reduce them into 5, 6 or 7 ideas without losing the complexity involved. Thermodynamics isn’t a very hard chapter normally., but it can get confusing when there are a million ways to find enthalpy. The key is that you are sometimes meant to choose ONE of these and sometime meant to use TWO of these. Always based on CONTEXT.
Here, then, are the 7 golden rules of Thermochemistry for the AP and IB student.
1. There are many ways to measure enthalpy (also casually known as heat) of a reaction: Here they are numbered/labeled (a) – (f)
a) When there is a temperature change in a substance without phase change,
we use:
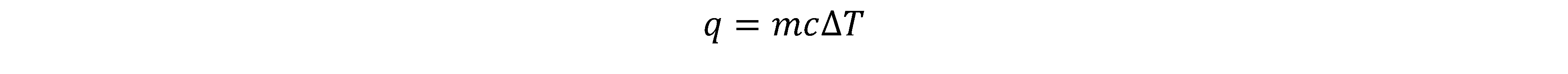
b) When there is a phase change, i.e., melting, we use: (For boiling, we use the enthalpy of vaporization also in J/g)
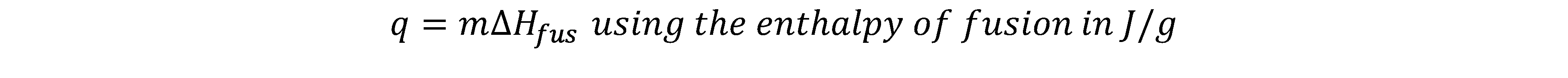
c) When you combine these two concepts is when you get heating and cooling curves.
d) When there are many reactions, we add them up in linear combinations to get our final equation. This is called Hess’s law. Remember systems of equations from Algebra 1,2?
e) You could also use formation enthalpies to find the enthalpy of a reaction. For eg.

f) Finally, we use bond enthalpies. Bond enthalpies or bond dissociation energies are the energies required to break a bond.

2) A formation reaction is quite literally the formation of a substance from its elements in their most natural state. Therefore, the formation enthalpy of an element will be 0 (As long as it is in its most natural state).
3) All of these reactions above tie into the first law of Thermodynamics: the law of conservation of energy.
If that is not clear, note that the primary concept we use in any thermodynamic problem that we don’t understand is this: heat lost = heat gained.
This heat may be calculated in any of the 5 ways above.
4) The second law of thermodynamics talks about entropy of the universe and how it increases with every spontaneous process. BUT, to measure spontaneity of a reaction, we can’t measure the entropy change of the universe every time. Which is where the idea of free energy comes in.
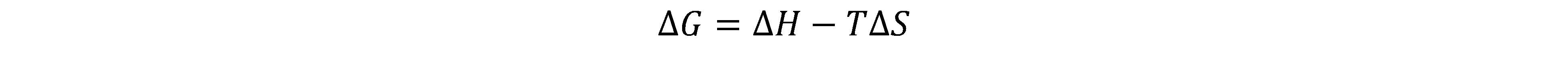
5) If enthalpy is negative, that is, exothermic, it contributes to the spontaneity of a reaction. If entropy is positive, it contributes to the spontaneity of a reaction. If they both don’t point the same way, it depends on their values and the temperature.
6) The free energy ΔG is linked closely to the equilibrium constant.
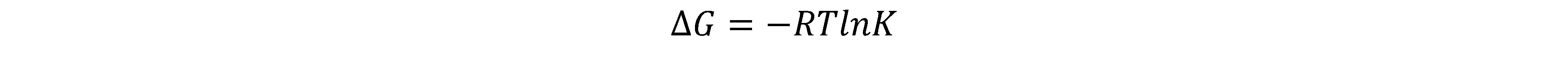
7) Remember that not only ΔH, but also ΔG and ΔS can be calculated from their formation values just as:



