This is Part 2 of Acids and Bases, with a detailed but very very short 5 point reveiw of the dreaded neutralization reactions.
For Part 1, Click here.
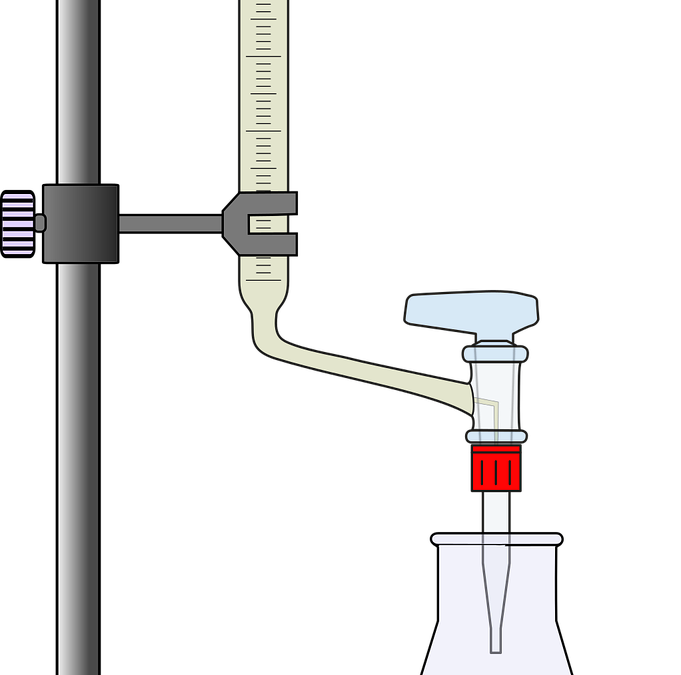
What is a titration?
Very simply, it is a neutralization reaction between an acid and a base.
What is a neutralization?
When equal moles of acid and base react with one another to stoichiometric completion to form salt and water.

Does that mean the solution is neutral?
NO. Unfortunately, no. It only means that .
The solution will be:
- neutral if both acid ad base are strong
- basic if acid is weak and base is strong
- acidic if acid is strong and base is weak
How do you solve these equations?
Using stoichiometry, because neutralizations are SINGLE ARROW REACTIONS. Then find out what the product is. You have 3 choices.
You are left with:
- HA and NaA: this is a buffer. Use Hendersen Hasselbach.
- Only NaA: this is a basic solution if HA is a weak acid. Use ICE.
If you only end up with a conjugate acid, also use ICE.
What about titration curves?
The inflection point of the titration curve represents “neutralization” and will be acidic/basic/neutral as stated above.