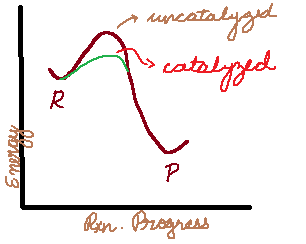
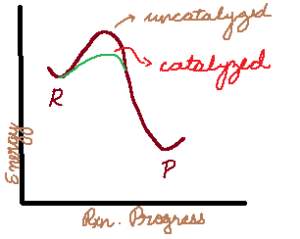
Reaction Mechanisms (the small steps that chemical reactions are broken down into) are a big part of Kinetics. And Catalysts and Intermediates are a small part of mechanisms.
You may have heard that a catalyst speeds up a reaction, but is not used up during the reaction. The truth is that a catalyst is used up but regenerated.
How then is this different from an intermediate? An intermediate is generated and THEN used up. Note the difference in the order.
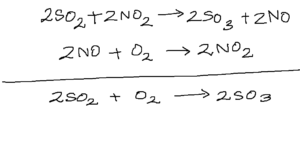 In the picture on the right, can you guess the nature of the catalyst and the intermediate?
In the picture on the right, can you guess the nature of the catalyst and the intermediate?
To relieve you of the suspense, the catalyst is and the intermediate is
.
What else do you need to know about catalysts and intermediates?
- Catalysts lower the activation energy, and this is the ONLY way that they speed up the reaction, by providing a shortcut for the reaction to go through.
- Intermediates on the other hand show their significance when calculating rate laws.If in the mechanism on the right, I told you that the second step was the rate determining step, and the first step was a fast step,
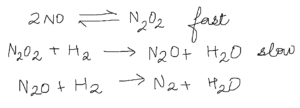
you would come up with the Rate Law,
However, intermediates cannot appear in rate equations.Using the first fast step, we substitutefor
, making the rate law,
And that is generally the end of the last part of the chapter on Chemical Kinetics. Feel free to write on facebook.com/plumpuddingchem if you have questions.