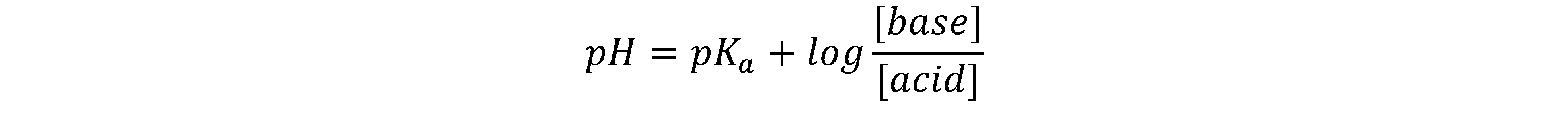1. The importance of pH and the many ways to find it.
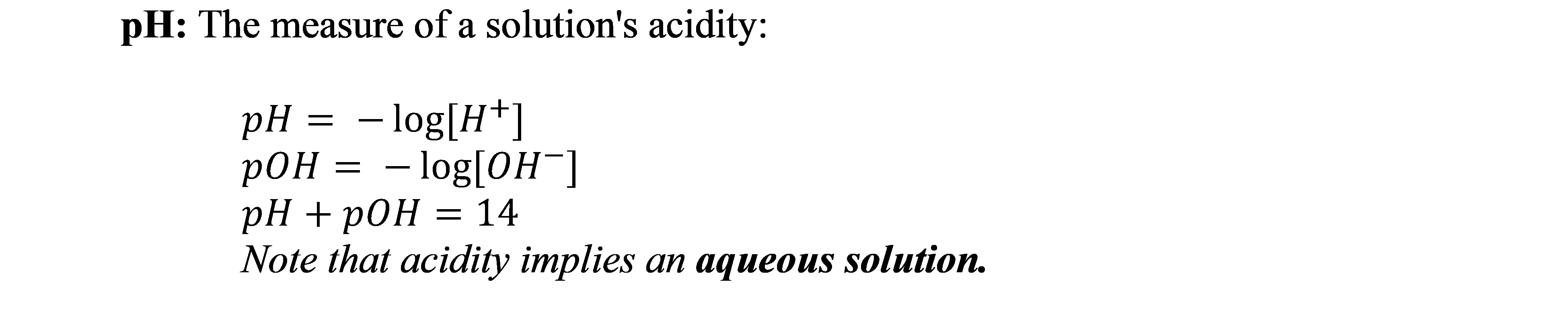
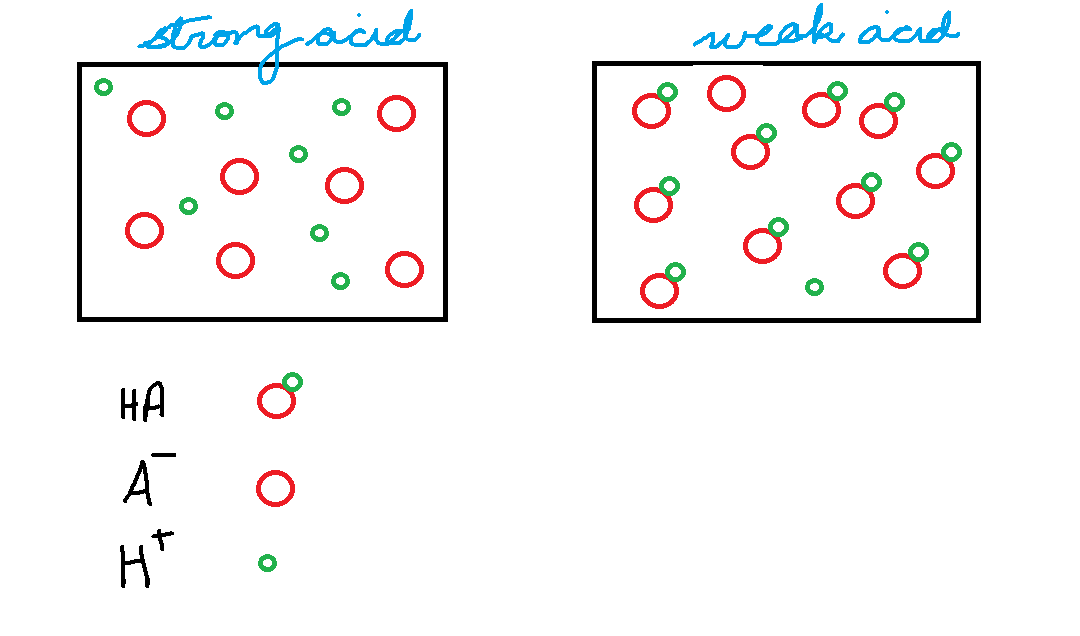
2. There are weak acids and strong acids.
Strong acids dissociate completely. What’s that? This process is known as acid dissociation:
This is what makes an acid acidic, i.e., release of an H+
Again, this means that in solution, there is not a single molecule of HA, every molecule has dissociated.
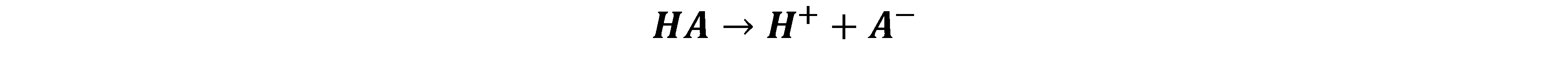
Weak acids dissociate partially.
This means that 95-99.99 % of the weak acids are in the form of HA.
These are equilibrium reactions: double arrow↔.
And it means an equilibrium constant called the ACID DISSOCIATION CONSTANT, Ka, is involved.


3. To find [H+] in a strong acid solution, you just use stoichiometry. nHA = nH+, where n is the number of moles.
To find [H+] in a weak acid solution, you use an ICE table. Eg.
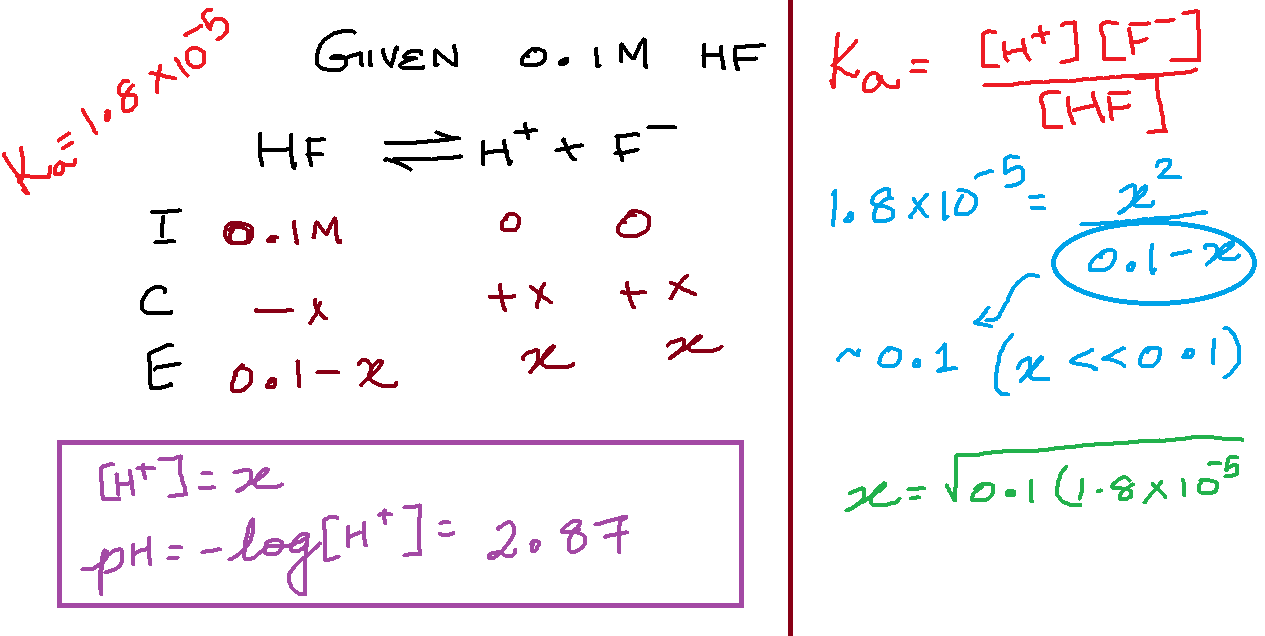
4. ALL acids (HA) dissociate to form their conjugate bases (A–).
And the stronger the acid, the weaker the conjugate base.
Conjugate Bases often come attached to a metal ion, like Na+, and look like NaA.
Remember, NaA, KA, etc are just A– in disguise and if we are talking of a weak conjugate base, you need an ICE equation to solve for pH of a given base.
5. A combination of a weak acid and its conjugate base is called a buffer.
So HA and A–.
And buffer pHs are solved by the Henderson Hasselbach equation
(also given in your equation sheet).