This is a semi- solved problem from the 2014 AP Chemistry Free Response section: Q2. The idea is to give the student (you) some clues to solve the problem without completely solving it for you.
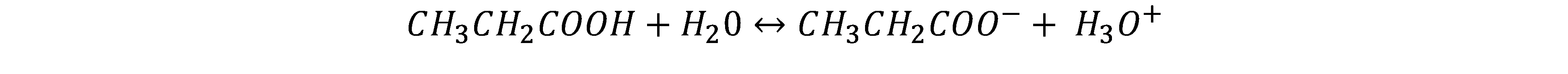
(a) learly, the acid is whatever loses a proton (or H+ or H3O+ in disguise), i.e., CH3COOH and the conjugate base is what it becomes: CH3COO–
(b) Hint: Set up an ICE equation with the equation on the left.
Remember that pH can give you the concentration of H3O+ to use in the ICE equation.

(i) Hint:
You have been given equal moles of propanoic acid and NaOH.
(Remember M*V = number of moles)
Does that mean pH =7 (neutrality)?
Not if this is a reaction between a weak acid and a strong base, in which case the salt will be basic.
(ii) Hint: The concept is yet again a contrast between a strong acid and a weak acid.
Imagine a strong acid as generous, completely dissociating to give protons or H+.
A weak acid, on the hand, is miserly, letting go of less than 5% of its protons.
It stands to reason that we will need a whole lot more of the propanoic acid than the HCl to attain the same pH.

Hint: This is a neutralization reaction. And neutralization reactions are always “single arrow” to be solved by stoichiometry.
The first part is your standard
MaVa=MbVb
Remember that in an earlier step you had found the Ka of the acid. Compare pKas and decide if you need a different indicator.

